The measurable heat flux that accompanies active transport by Ca2+-ATPase
Abstract
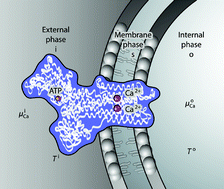
We present a new mesoscopic basis which can be used to derive flux equations for the forward and reverse mode of operation of ion-pumps. We obtain a description of the fluxes far from global equilibrium. An asymmetric set of transport coefficients is obtained, by assuming that the chemical reaction as well as the ion transports are activated, and that the enzyme has a temperature independent of the activation coordinates. Close to global equilibrium, the description reduces to the well known one from non-equilibrium thermodynamics with a symmetric set of transport coefficients. We show how the measurable heat flux and the heat production under isothermal conditions, as well as thermogenesis, can be defined. Thermogenesis is defined via the onset of the chemical reaction or ion transports by a temperature drop. A prescription has been given for how to determine transport coefficients on the mesocopic level, using the macroscopic coefficient obtained from measurements, the activation enthalpy, and a proper probability distribution. The method may give new impetus to a long-standing unsolved transport problem in biophysics.

 Please wait while we load your content...
Please wait while we load your content...