Breakdown of hydration repulsion between charged surfaces in aqueous Cs+ solutions
Abstract
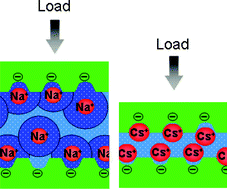
Using a surface force balance, we have measured the normal and shear forces between mica surfaces across aqueous caesium salt solutions (CsNO3 and CsCl) up to 100 mM concentrations. In contrast to all other
- This article is part of the themed collection: Water at interfaces
 Please wait while we load your content...
Please wait while we load your content...