”Developing paradigms of chemical bonding: adaptive natural density partitioning
Abstract
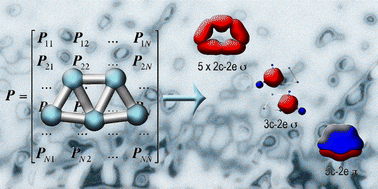
A method of description of the chemical bonding combining the compactness and intuitive simplicity of Lewis theory with the flexibility and generality of canonical molecular orbital theory is presented, which is called adaptive natural density partitioning. The objects of chemical bonding in this method are n-center 2-electron bonds, where n goes from one (lone-pair) to the maximum number of atoms in the system (completely delocalized bonding). The algorithm is a generalization of the natural bonding orbital analysis and is based on the diagonalization of the blocks of the first-order density

 Please wait while we load your content...
Please wait while we load your content...