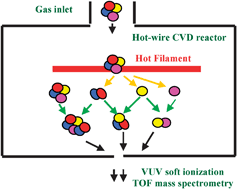
To study the effect of an Si–Si bond on gas-phase reaction chemistry in the hot-wire chemical vapor deposition (HWCVD) process with a single source alkylsilane molecule, soft ionization with a vacuum ultraviolet wavelength of 118 nm was used with time-of-flight mass spectrometry to examine the products from the primary decomposition of hexamethyldisilane (HMDS) on a heated tungsten (W) filament and from secondary gas-phase reactions in a HWCVD reactor. It is found that both Si–Si and Si–C bonds break when HMDS decomposes on the W filament. The dominance of the breakage of Si–Si over Si–C bond has been demonstrated. In the reactor, the abstraction of methyl and H atom, respectively, from the abundant HMDS molecules by the dominant primary trimethylsilyl radicals produces tetramethylsilane (TMS) and trimethylsilane (TriMS). Along with TMS and TriMS, various other alkyl-substituted silanes (m/z = 160, 204, 262) and silyl-substituted alkanes (m/z = 218, 276, 290) are also formed from radical combination reactions. With HMDS, an increasing number of Si–Si bonds are found in the gas-phase reaction products aside from the Si–C bond which has been shown to be the major bond connection in the products when TMS is used in the same reactor. Three methyl-substituted 1,3-disilacyclobutane species (m/z = 116, 130, 144) are present in the reactor with HMDS, suggesting a more active involvement from the reactive silene intermediates.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...