LIF studies of iodine oxide chemistry
Part 3.† Reactions IO + NO3 → OIO + NO2, I + NO3 → IO + NO2, and CH2I + O2 → (products): implications for the chemistry of the marine atmosphere at night
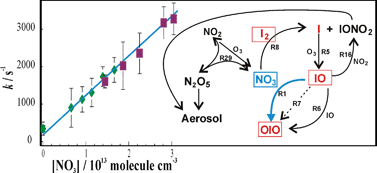
Abstract
The technique of pulsed laser photolysis coupled to

 Please wait while we load your content...
Please wait while we load your content...