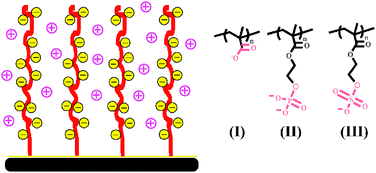
The present work reports on a systematic study of the swelling/collapse transition of three anionic polymer brushes bearing carboxylate, phosphate and sulfonate side groupsviaAFM measurement. Time scale of conformation change process can be approximately evaluated directly. All the three brushes in their sodium salt forms stretch away from the surface in pure water, as a result of charge repulsion and uptake of water. The collapse of weak brushes has two ways: normal charge screening and precipitation (strong ion pairing), depending on the types of cations that have different coordination capabilities with anionic groups. Alkali metal ions can make brushes shrink only at relatively high concentrations following a gradually increased charge screening mechanism. The brushes collapsed in this way can be restored by simply flushing with water. However, multivalent cations can irreversibly collapse brushes more easily even under very low concentrations (<10−3 mol L−1). The brushes cannot be restored with simple water rinsing even for strong sulfonate containing brushes. In this case, recovery can be achieved by ion exchange in concentrated salt solution, which facilitates transit from strong ion pairing to less strong charge screening and then flushing with water. Alternatively, the multivalent ion can be extracted with chelating reagent of low concentration (10 mM EDTA). As the chelating agent doesn’t affect the conformation of brushes, the brushes are one-step recovered directly, much more efficient than with high concentration electrolyte which usually requires extra water rinsing to remove excess salt inside brushes. The interaction between anions in the brushes and metal ions represents a model system to profoundly understand the mechanism of bio-mimic motions and how muscle works. In this regard, the present study provides useful information for the development of polyelectrolyte brushes based ion sensor and ion powered nanoactuators.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...