Understanding the self-assembling process in crystalline cyclooctitols: an insight into the conformational flexibility of medium-sized rings†
Abstract
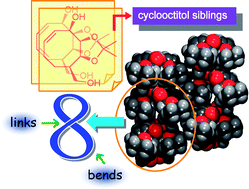
The crystal structures of two closely related cyclooctitols 1 and 2, in the form of their acetonide derivatives, have been analysed, in order to investigate the interrelationship between the preferred mode of

 Please wait while we load your content...
Please wait while we load your content...