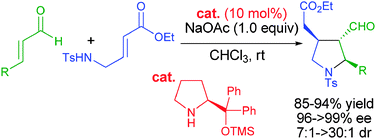
Highly enantio- and diastereoselective organocatalytic cascade aza-Michael–Michael reactions: a direct method for the synthesis of trisubstituted chiral pyrrolidines†
Abstract
An unprecedented highly enantio- and diastereoselective cascade aza-Michael–Michael reaction of α,β-unsaturated aldehydes with trans-γ-Ts protected

 Please wait while we load your content...
Please wait while we load your content...