Catalytic alkene cyclohydroamination via an imido mechanism†
Abstract
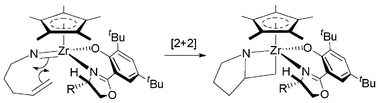
Chiral-at-metal half-sandwich diamide complexes catalyse enantioselective cyclohydroamination of aminoalkenes at unexpectedly high rates given their high coordination number and steric bulk; substantial evidence is presented which argues against the established σ-bond insertion process and is strongly indicative of an imido [2+2] cycloaddition mechanism.

 Please wait while we load your content...
Please wait while we load your content...