The internal structure of self-assembled peptide amphiphiles nanofibers†
Abstract
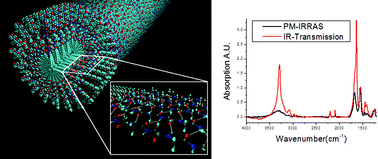
The self-assembly of peptide amphiphiles (PAs) into nanofibers and their bioactivity as well as physical properties have been investigated by our laboratory over the past few years. We report here on the use of transmission infrared spectroscopy (IR) and polarization modulation-infrared reflection-absorption spectroscopy (PM-IRRAS) to characterize the internal structure of the nanofibers. Depositing nanofibers flat on surfaces, and using the surface selection rules in PM-IRRAS, we demonstrate that peptide amphiphiles form β-sheets oriented parallel to the long-axis of nanofibers that pack radially from the nanofiber core. We show also that the extent of internal order depends on the molecular architecture and peptide sequence of PAs, with branched PAs yielding nanofibers with the lowest degree of internal order. Measurements of intensity and spectral position of the alkyl bands suggest that the hydrophobic core of these nanofibers can have internal order to an extent that correlates with order in their peptidic domains. We expect that bioactivity and physical properties will be controlled by the degree of internal order in these self-assembling nanostructures.

 Please wait while we load your content...
Please wait while we load your content...