Changes in tropospheric composition and air quality due to stratospheric ozone depletion and climate change†
Abstract
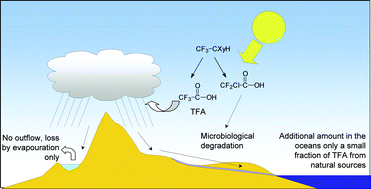
It is well-understood that reductions in air quality play a significant role in both environmental and human health. Interactions between ozone depletion and global climate change will significantly alter atmospheric chemistry which, in turn, will cause changes in concentrations of natural and human-made gases and aerosols. Models predict that tropospheric ozone near the surface will increase globally by up to 10 to 30 ppbv (33 to 100% increase) during the period 2000 to 2100. With the increase in the amount of the stratospheric ozone, increased transport from the stratosphere to the troposphere will result in different responses in polluted and unpolluted areas. In contrast, global changes in tropospheric hydroxyl radical (OH) are not predicted to be large, except where influenced by the presence of oxidizable organic matter, such as from large-scale forest fires. Recent measurements in a relatively clean location over 5 years showed that OH concentrations can be predicted by the intensity of solar ultraviolet radiation. If this relationship is confirmed by further observations, this approach could be used to simplify assessments of air quality. Analysis of surface-level ozone observations in Antarctica suggests that there has been a significant change in the chemistry of the boundary layer of the atmosphere in this region as a result of stratospheric ozone depletion. The oxidation potential of the Antarctic boundary layer is estimated to be greater now than before the development of the ozone hole. Recent modeling studies have suggested that iodine and iodine-containing substances from natural sources, such as the ocean, may increase stratospheric ozone depletion significantly in polar regions during spring. Given the uncertainty of the fate of iodine in the stratosphere, the results may also be relevant for stratospheric ozone depletion and measurements of the influence of these substances on ozone depletion should be considered in the future. In agreement with known usage and atmospheric loss processes, tropospheric concentrations of HFC-134a, the main human-made source of trifluoroacetic acid (TFA), is increasing rapidly. As HFC-134a is a potent greenhouse gas, this increasing concentration has implications for climate change. However, the risks to humans and the environment from substances, such as TFA, produced by atmospheric degradation of hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs) are considered minimal. Perfluoropolyethers, commonly used as industrial heat transfer fluids and proposed as chlorohydrofluorocarbon (CHFC) substitutes, show great stability to chemical degradation in the atmosphere. These substances have been suggested as substitutes for CHFCs but, as they are very persistent in the atmosphere, they may be important contributors to global warming. It is not known whether these substances will contribute significantly to global warming and its interaction with ozone depletion but they should be considered for further evaluation.
- This article is part of the themed collection: UNEP Report on Environmental Effects of Ozone Depletion

 Please wait while we load your content...
Please wait while we load your content...