Catalytic enantioselective stereoablative reactions: an unexploited approach to enantioselective catalysis†
Abstract
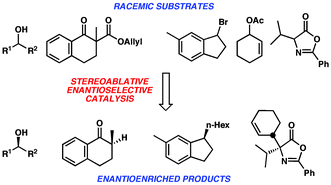
Approaches to the preparation of enantioenriched materials via catalytic methods that destroy stereogenic elements of a molecule are discussed. Although these processes often decrease overall molecular complexity, there are several notable advantages including material recycling, enantiodivergence and convergence, and increased substrate scope. Examples are accompanied by discussion of the critical design elements required for the success of these methods.

 Please wait while we load your content...
Please wait while we load your content...