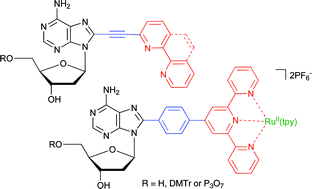
Synthesis of 2′-deoxyadenosine nucleosides bearing bipyridine-type ligands and their Ru-complexes in position 8 through cross-coupling reactions†‡
Abstract
The synthesis of the title 2′-deoxyadenosine derivatives bearing bipyridine, phenanthroline or terpyridine ligands and their corresponding RuII-complexes in position 8 linked via
- This article is part of the themed collection: Chemistry Nobel 2010 Web Collection: Cross-coupling reactions in organic chemistry

 Please wait while we load your content...
Please wait while we load your content...