Virtually epimerization-free synthesis of peptide-α-thioesters†
Abstract
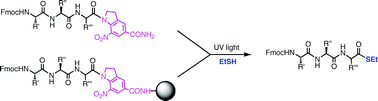
Under slightly basic or neutral reaction conditions peptide-α-thioesters are photochemically synthesized from peptide-α-nitroindoline precursors, either in solution, or by direct photorelease from a

 Please wait while we load your content...
Please wait while we load your content...