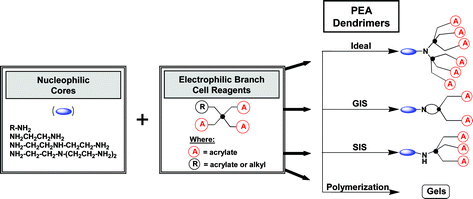
This work describes the Michael addition of unprotected, branched, (A)x-type acrylate monomers (i.e., trimethylolpropane triacrylate (TMPTA); x = 3 or pentaerythritol tetraacrylate (PTA); x = 4) to various mono- and poly-alkyleneamine cores under mild conditions to produce (core: amine); G = 1; [dendri-poly(ester-acrylate)z]; (PEA) type dendrimers in one step. Quite remarkably, this strategy did not necessitate large excess reagent protocols as required for traditional Tomalia-type poly(amidoamine) (PAMAM) dendrimer syntheses, yet produced relatively little oligomeric/polymeric side product. Ideal, mathematically predictable dendrimer structures were obtained in high yield with only modest excesses (i.e., 4.0 moles of monomer/core-NH); whereas, at lower ratios (i.e., 0.5–2.0 moles of monomer/core-NH), non-ideal, geometrically controlled dendrimers possessing, macrocyclic (looped) structures (i.e., geometrically induced stoichiometry (GIS)) were formed when adequate reactivity space was available on the amine scaffolding. However, when amine core reaction sites became highly congested, one observed the formation of well defined, non-ideal dendrimers exhibiting sterically induced stoichiometries (SIS). It is postulated that Michael addition of these nanoscale (i.e., 1–1.5 nm) branched (A)x monomers onto these sub-nanosized linear-α,ω-alkylenediamine or poly(alkyleneamine) scaffoldings produces a highly congested reaction environment even at this early generational state (i.e., G = 1). The observed products appear to be influenced and directed by these consequential steric and geometric space constraints.

 Please wait while we load your content...
Please wait while we load your content...