Structure and bonding in boron carbide: The invincibility of imperfections†
Abstract
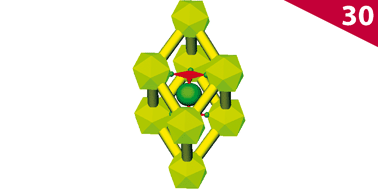
Boron carbide, usually described as B4C, has the mysterious ability to accommodate a large variation in carbon composition (to as much as B10C) without undergoing a basic structural change. We systematically explore how the bonding varies with carbon concentration in this structure and the origin of the fundamental electron deficiency of the phase. As the carbon concentration is reduced, we find that the exo-polyhedral BEq–C bonds of the icosahedra in the structure become increasingly engaged in multiple bonding, and the repulsive steric interactions between the bulky B12 units surrounding the carbon atom are reduced. The short bond lengths observed within the three-atom ![[triple bond splayed left]](https://www.rsc.org/images/entities/char_e00d.gif) C–B–C
C–B–C![[triple bond splayed right]](https://www.rsc.org/images/entities/char_e00c.gif) chains are then due to substantial π-bonding, while the carbon deficiency weakens its σ-framework significantly. We conclude that the idealized framework of boron carbide has to expel some electrons in order to maximize its bonding; disorder in the structure is an inevitable consequence of this partial oxidation. The localization of electronic states arising from the disorder leads to the semiconducting nature of boron carbide throughout its composition range.
chains are then due to substantial π-bonding, while the carbon deficiency weakens its σ-framework significantly. We conclude that the idealized framework of boron carbide has to expel some electrons in order to maximize its bonding; disorder in the structure is an inevitable consequence of this partial oxidation. The localization of electronic states arising from the disorder leads to the semiconducting nature of boron carbide throughout its composition range.

 Please wait while we load your content...
Please wait while we load your content...