Particle morphology, crystal orientation, and electrochemical reactivity of LiFePO4 synthesized by the hydrothermal method at 443 K
Abstract
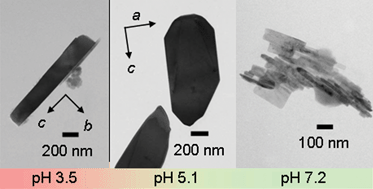
LiFePO4 (space group: Pnma) was synthesized by the hydrothermal method at 443 K. The pH of the precursor solution was systematically changed between 2.5 and 9.5. The particle morphology, crystal orientation, and electrochemical reactivity of the prepared LiFePO4 particles changed depending on the concentration of the Li source and pH of the precursor. The particles obtained from acidic solutions (pH ≈ 3.5) were needle-like particles. On the other hand, plate-like crystals were obtained from weak acidic solutions of 4 < pH < 6.5. At higher pH than 7.2, the particles became randomly shaped. The plate-like crystal had a large facet in the ac-plane, while the needle-like particles had a large facet in the bc-plane. The electrochemical properties of the prepared LiFePO4 were characterized in a mixed

 Please wait while we load your content...
Please wait while we load your content...