Dehydration behavior of the superprotonic conductor CsH2PO4 at moderate temperatures: 230 to 260 °C†
Abstract
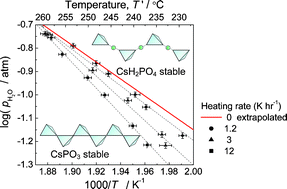
The dehydration behavior of caesium dihydrogen phosphate CsH2PO4 was investigated in the temperature range of 230 °C to 260 °C under high humidity, conditions of particular relevance to the operation of fuel cells based on this electrolyte. The onset temperature of dehydration was determined from changes in ionic conductivity on heating and confirmed by weight change measurements under isothermal conditions. The relationship between the onset temperature of dehydration (Tdehy) and water partial pressure (pH2O) was determined to be log(pH2O/atm = 6.11(±0.82) − 3.63(±0.42) × 1000/(Tdehy/K), from which the thermodynamic parameters of the dehydration reaction from CsH2PO4 to CsPO3 were evaluated. The dehydration pathway was then probed by X-ray powder diffraction analysis of the product phases and by thermogravimetric analysis under slow heating. It was found that, although the equilibrium dehydration product is solid caesium metaphosphate CsPO3, the reaction occurs via two overlapping steps: CsH2PO4 → Cs2H2P2O7 → CsPO3, with solid caesium hydrogen pyrophosphate, Cs2H2P2O7, appearing as a kinetically favored, transient phase.
- This article is part of the themed collection: New energy materials

 Please wait while we load your content...
Please wait while we load your content...