Independent verification of the saturation hydrogen uptake in MOF-177 and establishment of a benchmark for hydrogen adsorption in metal–organic frameworks†‡
Abstract
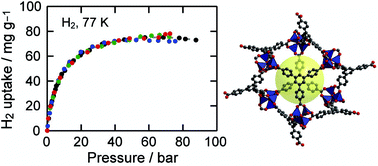
Hydrogen isotherms for MOF-177, Zn4O(1,3,5-benzenetribenzoate)2, crystals were independently measured by volumetric and gravimetric methods at 77 K to confirm its hydrogen uptake capacity and to establish the importance of calibrating gas adsorption instrumentation prior to evaluating H2 storage capacities. Reproducibility of hydrogen adsorption experiments is important because non-systematic errors in measurements can easily occur leading to erroneous reports of capacities. The surface excess weight percentage of hydrogen uptake in MOF-177 samples is 7.5 wt% at 70 bar, which corresponds to an absolute adsorbed amount of 11 wt%. These values are in agreement with our previous report and with those found independently by Southwest Research Institute. Considering its well-known structure and its significant H2 uptake properties, we believe MOF-177 is an excellent material to serve as a benchmark adsorber.
- This article is part of the themed collection: New energy materials

 Please wait while we load your content...
Please wait while we load your content...