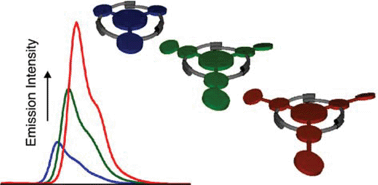
A highly modular and convergent synthetic route was devised to construct a series of planar π-conjugated molecules with systematically varied structural dimensions and electronic characteristics. High-yielding triple Schiff base condensation reactions between π-extended bulky anilines and 1,3,5-triformylphloroglucinol furnished a series of pseudo C3-symmetric tris(N-salicylideneamine)s displaying intense absorptions at λmax = 445–475 nm and emissions at λmax = 470–504 nm. X-Ray crystallographic studies revealed that intricate hydrogen-bonding networks sustain the planar conjugation of these discotic molecules, the HOMO–LUMO gaps of which decrease with increasing conjugation area. This reduction in excitation energy is accompanied by a nearly 4-fold enhancement in emission quantum yield (ΦF). Past a structural threshold, however, increasing conjugation area leads to either (i) decrease in ΦF or (ii) development of localized electronic transitions. These findings provide a well-defined structural window for future elaboration of this emerging family of dynamic 2-D conjugation, the luminescence properties of which have already been shown to reversibly change in response to external stimuli.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...