Carboxy-mesoporous carbon and its excellent adsorption capability for proteins†
Abstract
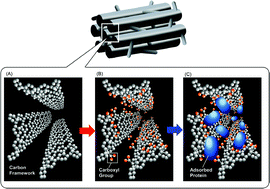
Here we report for the first time on the functionalization of hexagonally ordered mesoporous carbon through a simple oxidation using ammonium persulfate solution (APS). The degree of functionalization can be easily controlled by the simple adjustment of the oxidation parameters such as oxidation time, APS concentration and oxidation temperature. The functionalized materials have been unambiguously characterized by XRD, nitrogen adsorption, FT-IR and HR-FESEM measurements. It has been found that the functionalized mesoporous carbon, “carboxy mesoporous carbon”, shows two times higher adsorption capability for biomolecules than pristine mesoporous carbon. The higher protein adsorption capacity of carboxy mesoporous carbon may be due to the easy access of all the adsorption sites in the interior part of the mesopores because of the oxidation of small carbon rods which are normally oriented perpendicular to the primary mesopores of CMK-3 carbon material. We expect that this novel methodology could be used to create COOH functional groups on the surface of the mesoporous carbons with different structures which can further easily be modified with various functionalities such as amines, vitamins and chiral molecules. Moreover, these functionalized mesoporous carbon materials can be utilized as a solid and stable support for immobilizing chiral amine complexes and salen complexes, amino acids and nanoparticle fabrication and may also be applicable in drug delivery, separation and adsorption technology, column packing materials for chromatographic system and fuel cell anodic devices.

 Please wait while we load your content...
Please wait while we load your content...