Ionic liquids via reaction of the zwitterionic 1,3-dimethylimidazolium-2-carboxylate with protic acids. Overcoming synthetic limitations and establishing new halide free protocols for the formation of ILs
Abstract
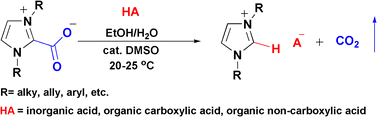
The previously reported preparation of 1,3-dimethylimidazolium salts by the reaction of 1,3-dialkylimidazolium-2-carboxylate zwitterions with protic acids has been reinvestigated in detail, leading to the identification of two competing reactions: isomerisation and decarboxylation. The ability to control both pathways allows this methodology to be used as an effective, green, waste-free approach to readily prepare a wide range of ionic liquids in high yields. Additionally, this reaction protocol opens new possibilities in the formation of other

 Please wait while we load your content...
Please wait while we load your content...