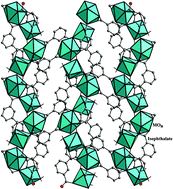
Two series of rare-earth isophthalates of the general formula, [M2(H2O)][{C6H4(COO)2}2{C6H4(COOH)(COO)}2]·H2O, M = La (I), Pr (Ia), and Nd (Ib) and [M2(H2O)2][{C6H4(COO)2}3]·H2O, M = Y (II), Gd (IIa), and Dy (IIb) have been prepared by the reaction of the corresponding trivalent lanthanide salts and isophthalic acid under mild hydrothermal conditions. The La (I), Pr (Ia) and Nd (Ib) have MO9 polyhedra connected to the isophthalate anions forming a two-dimensional structure, whereas Y (II), Gd (IIa) and Dy (IIb) have MO7 and MO8 polyhedral units connected to the isophthalate anions forming a different, but related two-dimensional structure. Both the structures are stabilized by hydrogen bonding and π⋯π/CH⋯π interactions. Partial substitution of Eu and Tb (2 and 4%) at the La (I) and Y (II) sites give rise to characteristic red/pink or green luminescence, indicating a ligand-sensitized metal-centered emission. The Nd (Ib) compound shows interesting UV and blue emission through an up-conversion process.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...