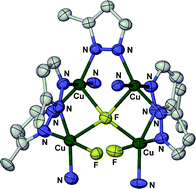
Treatment of CuF2 with 2 equiv of 3{5}-[pyrid-2-yl]pyrazole (HpzPy), 3{5}-phenylpyrazole (HpzPh) or 3{5}-[4-fluorophenyl]pyrazole (HpzPhF) in MeOH, followed by evaporation to dryness and recrystallisation of the solid residues, allows solvated crystals of [{Cu(µ-pzPy)(pzPy)}2] (1), [{Cu(µ-pzPh)2}4] (2) and [Cu4F2(µ4-F)(µ-pzPhF)5(HpzPhF)4] (3) to be isolated in moderate-to-good yields. Similar reactions of these three pyrazoles with Cu(OH)2 in refluxing MeOH respectively afford 1, 2 and [Cu(pzPhF)2(HpzPhF)2] (4) in ca. 10% yield. Crystalline 1·1/2H2O·2CHCl3 contains two independent dinuclear molecules with a puckered di-(1,2-pyrazolido) bridge motif, linked by a bridging, hydrogen-bonding water molecule. Compound 2·1/2C5H12, containing cyclic, square tetranuclear complex molecules, is the first homoleptic divalent metal pyrazolide to have a discrete molecular rather than polymeric structure, for a metal other than Pd or Pt. The two independent complex molecules in 3·3/4CH2Cl2·HpzPhF contain a unique tetrahedral [Cu4(µ4-F)]7+ core, three of whose edges are spanned by bridging pyrazolido groups. Magnetic data show that the copper centres in 1 and 3 are antiferromagnetically coupled, but that dried bulk samples of 2 do not retain their molecular structure.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...