A cadmium hydroxide complex of a N3S-donor ligand containing two hydrogen bond donors: synthesis, characterization, and CO2 reactivity
Abstract
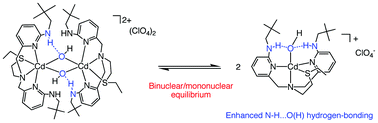
Treatment of the ebnpa (
- This article is part of the themed collection: Carbon dioxide at metal centres

 Please wait while we load your content...
Please wait while we load your content...