Structures and energetics of 98 atom Pd–Pt nanoalloys: potential stability of the Leary tetrahedron for bimetallic nanoparticles†
Abstract
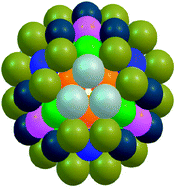
The energetics of 98 atom bimetallic Pd–Pt clusters are studied using a combination of: a genetic algorithm technique (to explore vast areas of the configurational space); a basin-hopping atom-exchange routine (to search for lowest-energy homotops at fixed composition); and a shell optimisation approach (to search for high symmetry isomers). The interatomic interactions between Pd and Pt are modelled by the Gupta many-body empirical potential. For most compositions, the putative global minima are found to have structures based on defective Marks decahedra, but in the composition range from Pd46Pt52 to Pd63Pt35, the Leary tetrahedron (LT)—a structure previously identified for 98 atom Lennard-Jones clusters—is consistently found as the most stable structure. Based on the excess energy stability criterion, Pd56Pt42 represents the most stable cluster across the entire composition range. This structure, a Td-symmetry LT, exhibits multi-layer segregation with an innermost core of Pd atoms, an intermediate layer of Pt atoms and an outermost Pd surface shell (Pd–Pt–Pd). The stability of the Leary tetrahedron is compared against other low-energy competing structural motifs: the Marks decahedron (Dh-M), a “quasi” tetrahedron (a closed-packed structure) and two other closed-packed structures. The stability of LT structures is rationalized in terms of their spherical shape and the large number of nearest neighbours.

 Please wait while we load your content...
Please wait while we load your content...