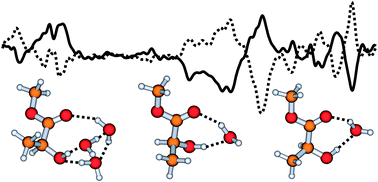
The infrared vibrational absorption (VA) and vibrational circular dichroism (VCD) spectra of methyl lactate were measured in the 1000–1800 cm−1 region in the CCl4 and H2O solvents, respectively. In particular, the chirality transfer effect, i.e. the H–O–H bending bands of the achiral water subunits that are hydrogen-bonded to the methyl lactate molecule exhibit substantial VCD strength, was detected experimentally. A series of density functional theory calculations using B3PW91 and B3LYP functionals with 6-311++G(d,p) and aug-cc-pVTZ basis sets were carried out to simulate the VA and VCD spectra of the methyl lactate monomer and the methyl lactate–(H2O)n complexes with n = 1, 2, 3. The population weighted VA and VCD spectra of the methyl lactate monomer are in good agreement with the experimental spectra in CCl4. Implicit polarizable continuum model was found to be inadequate to account for the hydrogen-bonding effect in the observed VA and VCD spectra in H2O. The methyl lactate–(H2O)n complexes with n = 1, 2, 3 were used to model the explicit hydrogen-bonding. The population weighted VA and VCD spectra of the methyl lactate–H2O binary complex are shown to capture the main spectral features in the observed spectra in aqueous solution. The theoretical modeling shows that the extent of chirality transfer depends sensitively on the specific binding sites taken by the achiral water molecules. The observation of chirality transfer effect opens a new spectral window to detect and to model the hydrogen-bonding solvent effect on VCD spectra of chiral molecules.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...