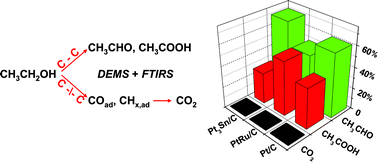
The interaction of colloid-based, carbon supported Pt/C (40 wt%), PtRu/C (45 wt%) and Pt3Sn/C (24 wt%) catalysts with ethanol and their performance for ethanol electrooxidation were investigated in model studies by electrochemical, in situ infrared spectroscopy and on-line differential electrochemical mass spectrometry measurements. The combined application of in situ spectroscopic techniques on realistic catalysts and under realistic reaction (DEMS, IR) and transport conditions (DEMS) yields new insight on mechanistic details of the reaction on these catalysts under the above reaction and transport conditions. Based on these results, the addition of Sn or Ru, though beneficial for the overall activity for ethanol oxidation, does not enhance the activity for C–C bond breaking. Dissociative adsorption of ethanol to form CO2 is more facile on the Pt/C catalyst than on PtRu/C and Pt3Sn/C catalysts within the potential range of technical interests (<0.6 V), but Pt/C is rapidly blocked by an inhibiting CO adlayer. In all cases acetaldehyde and acetic acid are dominant products, CO2 formation contributes less than 2% to the total current. The higher ethanol oxidation current density on the Pt3Sn/C catalyst at these potentials results from higher yields of C2 products, not from an improved complete ethanol oxidation to CO2.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...