Functional anion concept: effect of fluorine anion on hydrogen storage of sodium alanate†
Abstract
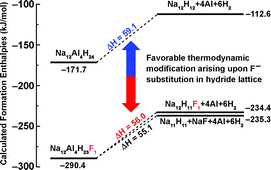
Doping NaAlH4 with Ti-catalyst has produced a promising hydrogen storage system that can be reversibly operated at moderate temperature conditions. Of the various dopant precursors, TiCl3 was well recognized due to its pronounced catalytic effect on the reversible dehydrogenation processes of sodium aluminium hydrides. Quite recently we experimentally found that TiF3 was even better than TiCl3 in terms of the critical hydrogen storage properties of the doped

 Please wait while we load your content...
Please wait while we load your content...