Vibrational overtone induced elimination reactions within hydrogen-bonded molecular clusters: the dynamics of water catalyzed reactions in CH2FOH·(H2O)n†
Abstract
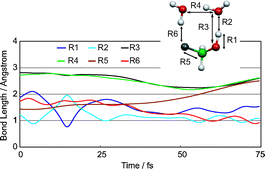
The dynamics of the light initiated OH-overtone induced elimination reactions CH2FOH·(H2O)n + hν
→ HF + CH2O + n(H2O), n = 1–3, are studied using classical trajectory simulations where the ab initio potential energy surface is computed “on-the-fly”. Hydrogen bonding to the water is found to lower the barrier to reaction by over 20 kcal mol−1 and modifies the mechanism to a concerted multiple H-atom transfer process. The reaction process is found to occur on a rapid timescale, <100 fs, and involves the hydronium ion as an intermediate. An essential aspect of dynamics is the successful competition of reaction with energy dissipation through

 Please wait while we load your content...
Please wait while we load your content...