Substituent effects on iminoboranes XBNH, HBNX and XBNX (X = H, CH3, NH2, OH, F) have been analyzed in the framework of the NBO, AIM and ELF approaches, using B3LYP/6-311++G(d,p) optimized geometries and electron densities. Boron-substituted derivatives, XBNH, are more stable than the corresponding nitrogen-substituted isomers HBNX, with the energy difference increasing as the electron withdrawing character of the substituent increases. The BN linkage is not much affected by N-substitution, but it is significantly altered when the substituent is attached to the boron atom in both XBNH and XBNX series of compounds. Moreover, substituent effects on the structures of iminoboranes are opposite those observed for the corresponding isoelectronic acetylene derivatives. The ELF analysis indicates that electron-withdrawing substituents enhance the localization of electrons in a torus around the CC or the BN axis. As a result, although electron density is depleted at the bcp, the bond does not necessarily become weaker, since density increases around the periphery, a phenomenon named the “hole” effect. The dissimilarities between acetylene and iminoborane derivatives are primarily a consequence of the significant distortion of this torus in the latter, due to the large difference between the electronegativities of B and N, which leads to a large contribution of the 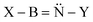 resonance structure in some cases. The “hole” effect is reflected in a reasonable correlation between the Laplacian of the electron density at the bcp and the BN bond length.
resonance structure in some cases. The “hole” effect is reflected in a reasonable correlation between the Laplacian of the electron density at the bcp and the BN bond length.
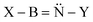 resonance structure in some cases. The “hole” effect is reflected in a reasonable correlation between the Laplacian of the electron density at the bcp and the BN bond length.
resonance structure in some cases. The “hole” effect is reflected in a reasonable correlation between the Laplacian of the electron density at the bcp and the BN bond length.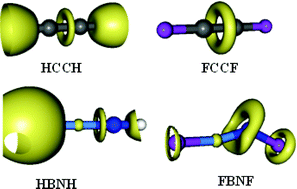

 Please wait while we load your content...
Please wait while we load your content...