On the σ,π-energy separation of the aromatic stabilization energy of cyclobutadiene
Abstract
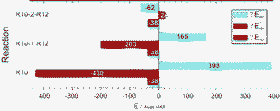
The separation of the aromatic stabilization energy (ASE) of cyclobutadiene (CBD) into a σ- and a π-component is reinvestigated. Eight different reactions are considered for this purpose. As expected, the total destabilization energies that result from these reactions depend only on the reference compound and not on the reaction itself. The heats of formation that can be obtained from the calculated reaction energies are in excellent agreement with the recently determined experimental value of 102.3 ± 3.8 kcal mol−1 (A. Fattahi, L. Liz, Z. Thian and S. R. Kass, Angew. Chem., Int. Ed., 2006, 45, 4984–4988). Evaluation of the angular strain in CBD from a newly considered reaction confirms earlier estimates and yields a strain energy of 34 ± 3 kcal mol−1. If referred to s-cis-butadiene this leads to an ASE of −37 ± 4 kcal mol−1 in close agreement with estimates provided by A. Fattahi, L. Liz, Z. Thian and S. R. Kass, Angew. Chem., Int. Ed., 2006, 45, 4984–4988; and by K. B. Wiberg, Chem. Rev., 2001, 101, 1317–1332. With s-trans-butadiene as reference we obtain −42 ± 4 kcal mol−1. This value is 8 to 10 kcal mol−1 less destabilizing than recent estimates of A. A. Deniz, K. S. Peters and G. J. Snyder, Science, 1999, 286, 1119–1112; and Kovačević, D. Barić, Z. B. Maksić, T. Müller, J. Phys. Chem. A, 2004, 108, 9126–9133. Attempts to separate ASE(CBD) and Estrain(CBD) into a σ- and a π-component do not lead to useful results. In contrast to ASE and Estrain themselves, the σ- and π-components depend strongly on the applied reaction. A detailed analysis reveals that it is not possible to associate these components with only one of the molecules that participate in the reaction. The components depend on all of these molecules and therefore on the underlying reaction. Generally, components that result from a formal σ,π-energy separation of aromatic stabilization energies or strain energies cannot be considered as the σ- and π-components of these energies.

 Please wait while we load your content...
Please wait while we load your content...