New chiral diamino-bis(tert-thiophene): an effective ligand for Pd- and Zn-catalyzed asymmetric transformations†
Abstract
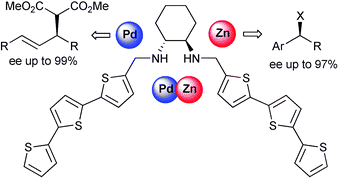
Enantiomerically pure diamino-bis(tert-thiophene) 1b proved to be a valuable and flexible chiral ligand for Pd- and Zn-catalyzed transformations, allowing for high levels of stereocontrol in asymmetric allylic alkylation (ee up to 99%) and hydrosilylations of prochiral carbonyls (ee up to 97%).

 Please wait while we load your content...
Please wait while we load your content...