Active site mutagenesis of the putative Diels–Alderase macrophomate synthase†
Abstract
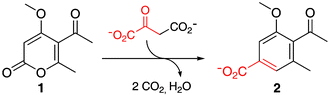
Although the macrophomate synthase active site is rich in potential functional groups, site-directed mutagenesis shows that only three residues are absolutely required for catalysis of oxaloacetate decarboxylation and trapping of the resulting enolate with a 2-pyrone; the other residues that line the binding pocket are surprisingly tolerant to substitution.

 Please wait while we load your content...
Please wait while we load your content...