A reductase-mimicking thiourea organocatalyst incorporating a covalently bound NADH analogue: efficient 1,2-diketone reduction with in situ prosthetic group generation and recycling†
Abstract
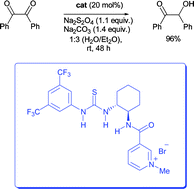
A new class of bifunctional organocatalyst promotes the chemoselective reduction of

 Please wait while we load your content...
Please wait while we load your content...