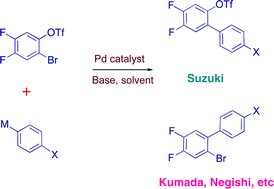
Aryl bromide/triflate selectivities reveal mechanistic divergence in palladium-catalysed couplings; the Suzuki–Miyaura anomaly†
Abstract
In palladium-catalysed cross-coupling reactions, the outcome of competition between aryl bromides and aryl triflates depends on the

 Please wait while we load your content...
Please wait while we load your content...