Directed evolution and axial chirality: optimization of the enantioselectivity of Pseudomonas aeruginosa lipase towards the kinetic resolution of a racemic allene†
Abstract
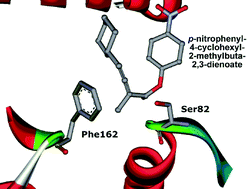
Directed evolution of Pseudomonas aeruginosa lipase by the use of combinatorial active site saturation test (CAST) criteria provided a highly enantioselective mutant (Leu162Phe) for kinetic resolution of an axially chiral

 Please wait while we load your content...
Please wait while we load your content...