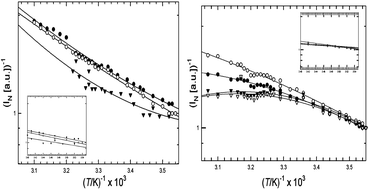
Dermal collagens have several fluorescent moieties in the UV and visible spectral regions that may serve as molecular probes of collagen. We studied the temperature-dependence of a commercial calf skin collagen and acid-extracted Skh-1 hairless mouse collagen at temperatures from 9 °C to 60 °C for excitation/emission wavelengths 270/305 nm (tyrosine), 270/360 nm (excimer-like aggregated species), 325/400 nm (dityrosine) and 370/450 nm (glycation adduct). L-tyrosine (1 × 10−5 M in 0.5 M HOAc) acted as a “reference compound” devoid of any collagen structural effects. In general, the fluorescence efficiency of these fluorophores decreases with increasing temperature. Assuming that rate constant for fluorescence deactivation has the form kd(T) = kdo exp (–ΔE/RT), an Arrhenius plot of log[(1/Φ) − 1] vs. 1/T affords a straight line whose (negative) slope is proportional to the activation energy, ΔE, of the radiationless process(es) that compete with fluorescence. Because it is difficult to accurately measure Φf for collagen-bound fluorophores, we derived an approximate formula for an activation parameter, ΔE*, evaluated from an Arrhenius-like plot of log 1/INvs. 1/T, (1/INvs. is the reciprocal normalized fluorescence intensity). Tyrosine in dilute solution affords a linear Arrhenius plot in both of the above cases. Using the known value of Φf = 0.21 for free tyrosine at room temperature, we determined that ΔE* is accurate to ∼25% in the present instance. Collagen curves are non-linear, but they are quasi-linear below ∼20 °C, where the helical form predominates. Values of ΔE* determined from the data at T < 20 °C ranged from 6.2–8.4 kJ mol−1 (1.5–2.0 kcal mol−1) for mouse collagen and 10.3–11.4 kJ mol−1 (2.5–2.7 kcal mol−1) for calf skin collagen, consistent with collisional deactivation of the fluorescent state via thermally enhanced molecular vibrations and rotations. Above 20 °C, log 1/INvs. 1/T plots from Skh-1 hairless mouse collagen are concave-downward, suggesting that fluorescence deactivation from the denatured coil has a significant temperature-independent component. For calf skin collagen, these plots are concave-upward, suggesting an increase in activation energy above Tm. These results suggest that collagen backbone and supramolecular structure can influence the temperature dependence of the bound fluorophores, indicating the future possibility of using activation data as a probe of supramolecular structure and conformation.

 Please wait while we load your content...
Please wait while we load your content...