Concise access to indolizidine and pyrroloazepine skeleta via intramolecular Schmidt reactions of azido 1,3-diketones†
Abstract
Readily prepared 2-alkyl-2-azidopropylcycloalkyl-1,3-diones undergo intramolecular Schmidt rearrangement with a range of hard Lewis acids, leading to indolizidinediones and pyrroloazepinediones. Chiral aluminium-based Lewis acids could also be used to mediate this symmetry-breaking transformation, but no significant asymmetric induction was observed.
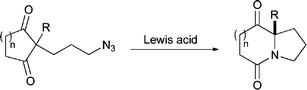

 Please wait while we load your content...
Please wait while we load your content...