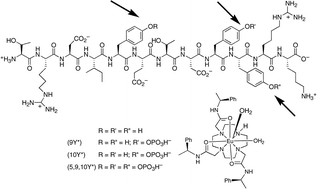
A cationic lanthanide complex binds selectively to phosphorylated tyrosine sites, aiding NMR analysis of the phosphorylated insulin receptor peptide fragment
Abstract
The binding of two cationic europium complexes to a differentially phosphorylated insulin receptor

 Please wait while we load your content...
Please wait while we load your content...