Two-directional synthesis and stereochemical assignment toward a C2 symmetric oxasqualenoid (+)-intricatetraol†
Abstract
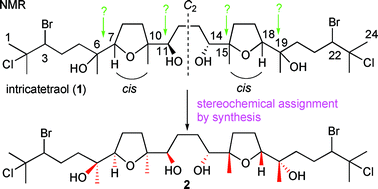
The asymmetric synthesis of tetraol (+)-3, a degradation product derived from a C2 symmetric oxasqualenoid intricatetraol 1, has been achieved through the two-directional synthesis starting from

 Please wait while we load your content...
Please wait while we load your content...