Chemical variation of natural product-like scaffolds: design and synthesis of spiroketal derivatives†
Abstract
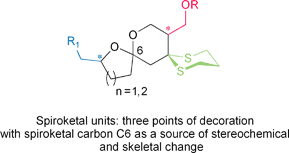
The design and synthesis of spiroketal structures and their chemical modification, leading to a collection of new small molecules for biological evaluation as orally-bioavailable lead compounds is described. Both [6,5]- and [6,6]-membered ring spiroketal units have been prepared in a stereochemically-varying fashion starting from commercially available (R)- or (S)-glycidol, in ten, eleven and twelve linear steps, in overall yields of 45, 40 and 20%, respectively. Further elaboration according to Lipinski's guidelines has given a collection of structurally-diverse, new spiroketal derivatives in high yields and with high purity.

 Please wait while we load your content...
Please wait while we load your content...