Selective manipulation of steroid hydroxyl groups with boronate esters: efficient access to antigenic C-3 linked steroid–protein conjugates and steroid sulfate standards for drug detection†‡
Abstract
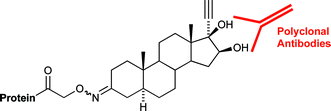
The temporary protection of 17α-alkyl-5α-androstane-3β,16β,17β triols as boronate esters is an efficient method for their regioselective functionalisation. This has been applied to the synthesis of protein–steroid conjugates 7–10 suitable for the development of immunoassays targeting classes of steroids banned from competition in Australian horse racing and other sports. The synthesis of steroids sulfate conjugates 42 and 44 for use as reference standards is also reported.

 Please wait while we load your content...
Please wait while we load your content...