Catalytic and stoichiometric approaches to the desymmetrisation of centrosymmetric piperazines by enantioselective acylation: a total synthesis of Dragmacidin A†
Abstract
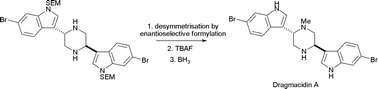
The enantioselective desymmetrisation of centrosymmetric piperazines was investigated using both catalytic and stoichiometric asymmetric acylation approaches. The catalytic approach involved the desymmetrisation of 2,5-trans-dimethylpiperazine under the control of chiral DMAP analogues. With one equivalent of piperazine, relative to the acylating agent, low yields of products were obtained in up to 70% ee. It was shown that an inevitable ‘proof reading’ effect was occurring which increased the enantiomeric excess of the desymmetrised product through its kinetic resolution. The desymmetrisation of centrosymmetric piperazines with chiral acylating agents [(1R,2R)-N-formyl-1,2-bis(pentafluorobenzenesulfonamido)cyclohexane and (1R,2R)-N-acetyl-1,2-bis(trifluoromethanesulfonamido)cyclohexane] was also studied. The yield and enantioselectivity of the process was highly dependent on the solvent used and the substitution of the piperazine. However, in some cases, good yields of enantiomerically enriched products could be obtained (up to 87% based on the limiting chiral reagent) in good enantiomeric excesses (up to 84% ee). The approach was exploited in the total synthesis of Dragmacidin A.

 Please wait while we load your content...
Please wait while we load your content...