Mass spectrometric identification of novel arsinothioyl-sugars in marine bivalves and algae
Abstract
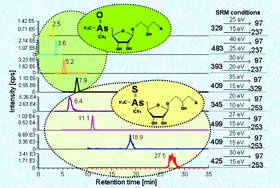
Following recent reports on the detection of the new group of thioarsenosugars in marine molluscs we have developed two chromatographic methods in order to explore the presence of additional species of this group in biological samples. A series of thioarsenosugar standards, containing four different thioarsenosugars in total, was prepared and used for method optimization. First, conventional anion exchange chromatographic conditions were modified to achieve efficient elution of the thioarsenosugars. This required relatively high contents of methanol in the mobile phase (up to 40%). Online hyphenation with electrospray tandem mass spectrometry provided good quality product ion mass spectra for the thioarsenosugars. These allowed the generation of collision induced dissociation breakdown curves for each compound which finally resulted in the development of a very sensitive and specific selected reaction monitoring approach. However, the high amount of methanol used with this method was not optimum for direct hyphenation with inductively coupled plasma mass spectrometry (ICP-MS) without a specially adapted interface. Therefore, reversed phase anion pairing HPLC with a low methanol content (5%) in the mobile phase was developed and used in this case. The combined application of both analytical techniques revealed the presence of two novel thioarsenosugars in marine bivalves and marine algae. Moreover, this is the first time that the detection of thioarsenosugars in marine algae is reported.

 Please wait while we load your content...
Please wait while we load your content...