CO2 absorption by aqueous NH3 solutions: speciation of ammonium carbamate, bicarbonate and carbonate by a 13C NMR study
Abstract
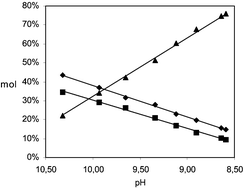
The absorption of CO2 in aqueous NH3 solutions occurs with high efficiency and loading capacity at room temperature and atmospheric pressure producing the ammonium salts of bicarbonate (HCO3−), carbonate (CO32−), and carbamate (NH2CO2−) anions. 13C NMR spectroscopy at room temperature has been proven to be a simple and reliable method to investigate the speciation in solution of these three ionic species. Fast equilibration of HCO3−/CO32− anions results in a single NMR peak whose chemical shift depends on the relative concentration of the two species. A method has been developed to correlate the chemical shift of this carbon resonance to the ratio of the two anionic species. Integration of the carbamate carbon peak provided the relative amount of this species with respect to HCO3−/CO32− pair. No other species was detected in solution by 13C NMR, and no solid compounds separated out under our experimental conditions. Finally, the relative amount of HCO3−, CO32−, and NH2CO2− in solution have been correlated to the molar ratio between free ammonia in solution and absorbed CO2.

 Please wait while we load your content...
Please wait while we load your content...