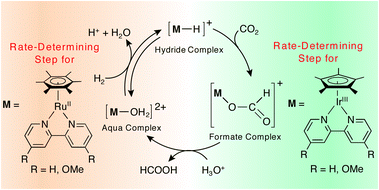
Ruthenium aqua complexes [(η6-C6Me6)RuII(L)(OH2)]2+ {L = bpy (1) and 4,4′-OMe-bpy (2), bpy = 2,2′-bipyridine, 4,4′-OMe-bpy = 4,4′-dimethoxy-2,2′-bipyridine} and iridium aqua complexes [Cp*IrIII(L)(OH2)]2+ {Cp* = η5-C5Me5, L = bpy (5) and 4,4′-OMe-bpy (6)} act as catalysts for hydrogenation of CO2 into HCOOH at pH 3.0 in H2O. The active hydride catalysts cannot be observed in the hydrogenation of CO2 with the ruthenium complexes, whereas the active hydride catalysts, [Cp*IrIII(L)(H)]+ {L = bpy (7) and 4,4′-OMe-bpy (8)}, have successfully been isolated after the hydrogenation of CO2 with the iridium complexes. The key to the success of the isolation of the active hydride catalysts is the change in the rate-determining step in the catalytic hydrogenation of CO2 from the formation of the active hydride catalysts, [(η6-C6Me6)RuII(L)(H)]+, to the reactions of [Cp*IrIII(L)(H)]+ with CO2, as indicated by the kinetic studies.

 Please wait while we load your content...
Please wait while we load your content...