Redox behavior of tumor-inhibiting ruthenium(iii) complexes and effects of physiological reductants on their binding to GMP†
Abstract
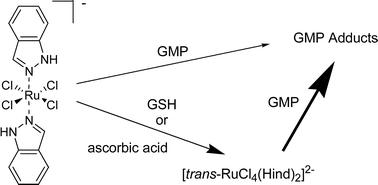
Biotransformation of ruthenium(III) anticancer complexes as hypothesized in the activation-by-reduction theory is the central topic of the present paper. The redox behavior of tetrachlorobis(azole)ruthenate(III)-type complexes was studied by NMR spectroscopy and square wave voltammetry. The influence of reducing agents on the binding behavior toward the DNA-modeling nucleotide GMP was determined by capillary electrophoresis, accompanied by identification of arising peaks by online coupling to electrospray ionization mass spectrometry. The determination of redox potentials revealed that the biologically relevant reductants ascorbic acid and glutathione are capable of reducing the studied Ru(III) complexes under physiological conditions. Characteristic differences in reduction kinetics dependent on the pH value can be explained by higher reduction strength of ascorbic acid and glutathione at higher pH compared to the pH-independent redox response of ruthenium(III) complexes. Binding behavior of (H2ind)[trans-RuCl4(Hind)2] (Hind = 1H-indazole) toward GMP was found to be increased upon addition of two equivalents of glutathione but not of ascorbic acid. In contrast, only a minor influence on the GMP-binding under reductive conditions was found for (H2im)[trans-RuCl4(Him)2] (KP418, Him = 1H-imidazole).
- This article is part of the themed collection: Celebrating 50 years of Dalton Transactions: Our Top 50

 Please wait while we load your content...
Please wait while we load your content...