Two families of variously-substituted N-pyrrolylphosphino-N′-arylaldimine ligands, 2-(aryl–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C4H3N–PR2 {3a–d R = Ph; 4a–d R = Pri2N}, have been prepared from the corresponding pyrrolylaldimines 2. The donor characteristics/basicity of P–N-chelating 3 and 4 have been assessed using a combination of 31P{1H} NMR and IR spectroscopies through study of the magnitudes of 1JSeP for the phosphorus(V) selenides 7 and 8, and measurement of νCO for the complexes [RhCl(CO)(3,4-κ2-P,N)] (5, 6), respectively. The synthesis of the palladium(II) complexes [PdCl2(3,4-κ2-P,N)] (9, 10) was readily achieved from reaction of 3 or 4 with [PdCl2(MeCN)2] in CH2Cl2. X-Ray crystallographic studies of 13d and 14b confirm the chelating nature of the P–N ligands, which adopt a distorted ‘envelope’ conformation, and highlight the potentially significant steric demands of these metal scaffolds. Reaction of equimolar quantities of 3 with [NiBr2(DME)] in MeCN afforded [NiBr2(3-κ2-P,N)] (15), while the same reaction undertaken in CH2Cl2 with 3c gave rise to the homoleptic bis(pyrrolatoimine) derivative [Ni{2-(mes–N
CH)C4H3N–PR2 {3a–d R = Ph; 4a–d R = Pri2N}, have been prepared from the corresponding pyrrolylaldimines 2. The donor characteristics/basicity of P–N-chelating 3 and 4 have been assessed using a combination of 31P{1H} NMR and IR spectroscopies through study of the magnitudes of 1JSeP for the phosphorus(V) selenides 7 and 8, and measurement of νCO for the complexes [RhCl(CO)(3,4-κ2-P,N)] (5, 6), respectively. The synthesis of the palladium(II) complexes [PdCl2(3,4-κ2-P,N)] (9, 10) was readily achieved from reaction of 3 or 4 with [PdCl2(MeCN)2] in CH2Cl2. X-Ray crystallographic studies of 13d and 14b confirm the chelating nature of the P–N ligands, which adopt a distorted ‘envelope’ conformation, and highlight the potentially significant steric demands of these metal scaffolds. Reaction of equimolar quantities of 3 with [NiBr2(DME)] in MeCN afforded [NiBr2(3-κ2-P,N)] (15), while the same reaction undertaken in CH2Cl2 with 3c gave rise to the homoleptic bis(pyrrolatoimine) derivative [Ni{2-(mes–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C4H3N}2] (16) in 45% yield, following P–N bond cleavage. Complex 16c was characterised in the solid-state by X-ray crystallography. No identifiable metal-containing complexes could be obtained on reaction of 4 with a variety of sources of Ni(II). The palladium dichloride complexes 13 and 14 proved inactive in combination with MAO or EtAlCl2 for ethylene polymerisation, and with methanesulfonic acid for CO/ethylene co-polymerisation. Contrastingly, the nickel complexes 15 in combination with 4.5 eq. EtAlCl2 catalysed the formation of butenes and hexenes with moderate activity from ethylene at 1 bar.
CH)C4H3N}2] (16) in 45% yield, following P–N bond cleavage. Complex 16c was characterised in the solid-state by X-ray crystallography. No identifiable metal-containing complexes could be obtained on reaction of 4 with a variety of sources of Ni(II). The palladium dichloride complexes 13 and 14 proved inactive in combination with MAO or EtAlCl2 for ethylene polymerisation, and with methanesulfonic acid for CO/ethylene co-polymerisation. Contrastingly, the nickel complexes 15 in combination with 4.5 eq. EtAlCl2 catalysed the formation of butenes and hexenes with moderate activity from ethylene at 1 bar.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C4H3N–PR2 {3a–d R = Ph; 4a–d R = Pri2N}, have been prepared from the corresponding pyrrolylaldimines 2. The donor characteristics/basicity of P–N-chelating 3 and 4 have been assessed using a combination of 31P{1H}
CH)C4H3N–PR2 {3a–d R = Ph; 4a–d R = Pri2N}, have been prepared from the corresponding pyrrolylaldimines 2. The donor characteristics/basicity of P–N-chelating 3 and 4 have been assessed using a combination of 31P{1H} ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C4H3N}2] (16) in 45% yield, following P–N bond cleavage. Complex 16c was characterised in the solid-state by
CH)C4H3N}2] (16) in 45% yield, following P–N bond cleavage. Complex 16c was characterised in the solid-state by 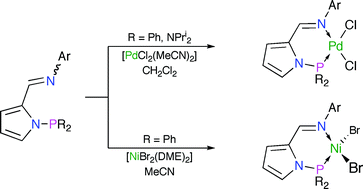

 Please wait while we load your content...
Please wait while we load your content...