Crystal structure of lead(ii) acetylacetonate and the structure of the acetylacetone solvated lead(ii) ion in solution studied by large-angle X-ray scattering†
Abstract
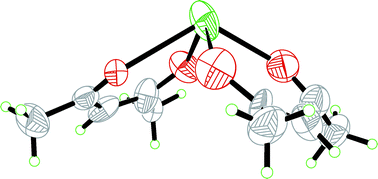
The crystal structure of bis(acetylacetonato)lead(II) and the structure of the acetylacetone solvated lead(II) ion in solution have been determined by

 Please wait while we load your content...
Please wait while we load your content...